We commonly refer to the interface between different phases as the interface, including gas/liquid, gas/solid, liquid/liquid, solid/solid, and solid/liquid. Among them, the interface containing a gas phase is referred to as the surface, including liquid surfaces and solid surfaces. Surfactants can change the chemical properties of the surface (interface) by forming an adsorption film through adsorption, reducing surface tension. Therefore, in simple terms, surfactants are a type of functional fine chemical product that, with a small amount added, can cause significant changes in the surface (interface) state. Thus, surfactants have functions such as wetting, penetration, foaming, emulsification, and sterilization.
So, how are these surfactant functions applied to the field of disinfection to enhance the effectiveness of disinfection? Let's first explore how the wetting and penetration functions contribute to improving the disinfection effect.
The majority of pathogenic microorganisms grow along with the formation of biofilms. Initially, planktonic microorganisms weakly adhere to the surface through forces such as van der Waals forces, hydrophobic interactions, and electrostatic forces. At this stage, the pathogenic microorganisms have not yet formed a stable and robust biofilm, making it relatively easy to remove. As the adhered pathogens grow and reproduce, they continuously secrete a gelatinous extracellular polysaccharide matrix, making their adhesion to the surface more robust, forming microbial colonies. The resulting biofilm transitions from thin to thick, making it challenging to remove.
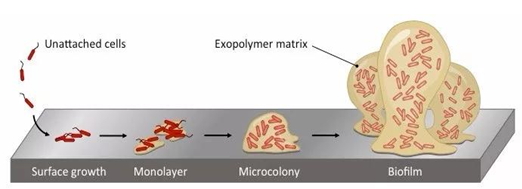
The polymeric matrix structure of extracellular polysaccharides and proteins in biofilms forms resistance, delaying the penetration of disinfectants and other substances into the biofilm matrix. Compared to directly acting on free-floating microorganisms, the presence of biofilms reduces the accessibility of disinfectants to microorganisms adhered to the surface.
In a comparative study of the resistance of bacterial biofilms and free-floating bacteria to disinfectants, it was found that using a disinfectant containing 800 mg/L of hydrogen peroxide, the average kill rates for planktonic forms of Burkholderia cepacia, Staphylococcus aureus, and Pseudomonas aeruginosa were 90.53%, 87.64%, and 83.16%, respectively. However, the average kill rates for their bacterial biofilms were only 82.56%, 84.81%, and 82.85%, respectively. When a disinfectant containing 150 mg/L of chlorine dioxide was applied for 1 minute, the average kill rates for planktonic forms of the same bacteria were 96.95%, 96.18%, and 96.05%, while the average kill rates for their bacterial biofilms were only 86.37%, 89.54%, and 91.20%, respectively. It can be seen that bacteria forming biofilms exhibit stronger resistance to disinfectants than free-floating bacteria.
Furthermore, some components of biofilm structures can react with certain disinfectants, causing partial consumption of the disinfectant and thereby reducing its disinfection efficacy. Additionally, the wetting coverage of disinfectants on the surface (interface) also affects their disinfection efficacy. Only when droplets completely cover the surface (interface) can the possibility of incomplete disinfection be truly avoided.
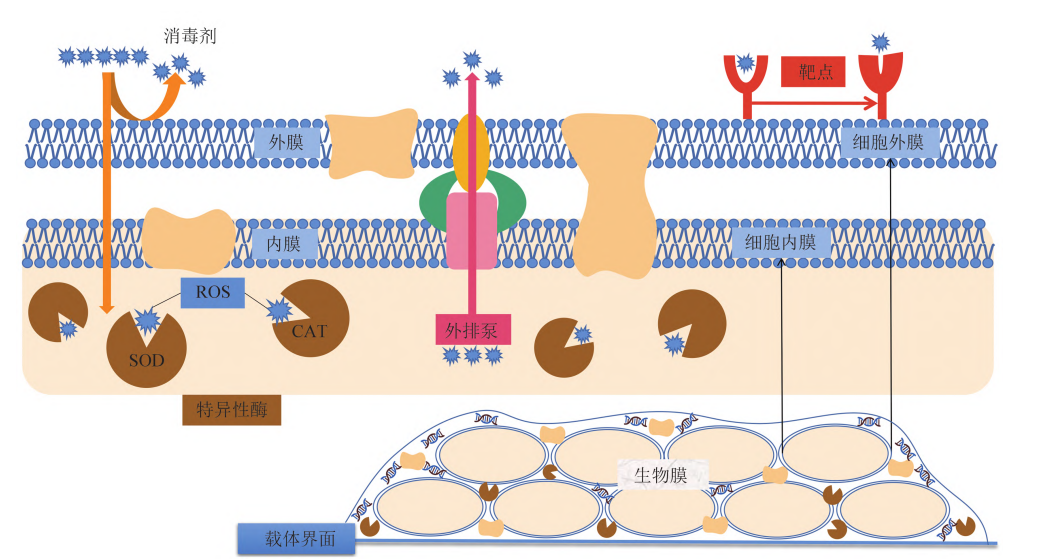
Based on the influence of biofilms and surface wetting on the disinfection effect, the wetting and penetration functions of surfactants can be utilized to enhance the disinfection effect through complexation with disinfectant components:
(1) Improvement of disinfection effect through wetting performance: The wetting function of surfactants enhances the wetting ability of disinfectants on the surface (interface), especially in areas prone to accumulation of pathogenic microorganism colonies, such as wall cracks and corners, which are difficult to clean. This ensures that the disinfectant achieves complete coverage of the disinfection interface without dead zones. On the other hand, wetting function allows the disinfectant to come into contact with the surface (interface) more rapidly. The combination of these two factors enhances the disinfection effect. The wetting degree of droplets on the surface (interface) can be assessed using the Dataphysics OCA15EC video-based optical contact angle measurement instrument (equipment in the Chengdu Kehongda Surfactant Research Center laboratory).
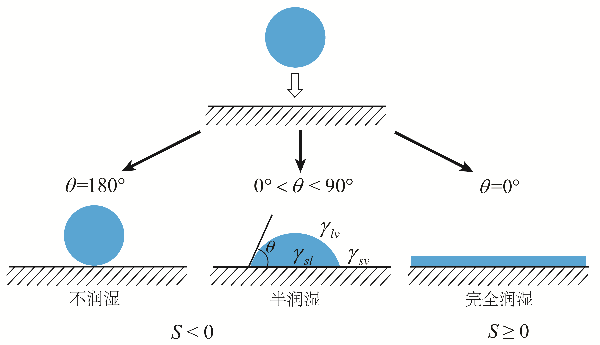
The correlation between contact angle size and wetting degree.
(2) Enhancement of Disinfection Effect through Penetration Performance: The penetration function of surfactants allows disinfectants to more rapidly disrupt the biofilm of pathogens, penetrate into the interior of the biofilm, and increase the contact between disinfectants and pathogenic microorganisms. Simultaneously, it assists disinfectants in more easily and quickly breaking down and penetrating the membrane structures of pathogenic cells, thereby enhancing the disinfection effect.
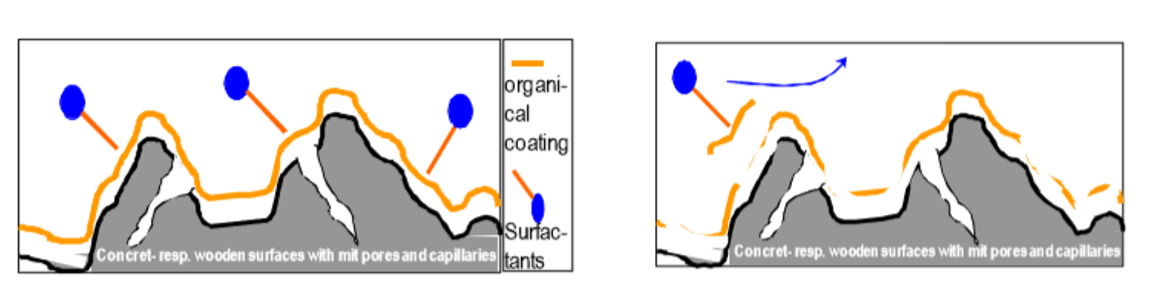
The penetration function of surfactants in disrupting biofilms.
In summary, surfactants, with their unique wetting and penetration functions, when combined with disinfectant components, can effectively enhance the contact between disinfectants and pathogenic microorganisms, thereby improving the disinfection effect.