Amine compounds constitute a significant portion of natural products, with all alkaloids containing nitrogen atoms. Amines are also frequently present in pharmaceuticals.
Due to the increased solubility from the amine's lone pair of electrons and the enhanced lipophilicity by substituents, amine drugs can possess the ability to penetrate cell membranes and the blood-brain barrier. Aliphatic amines are widely present in drug formulations, small molecule probes, and preclinical candidate drugs. Therefore, there is a continuous need for new and complex amines to explore reactivity, driving the development of innovative synthetic methods.
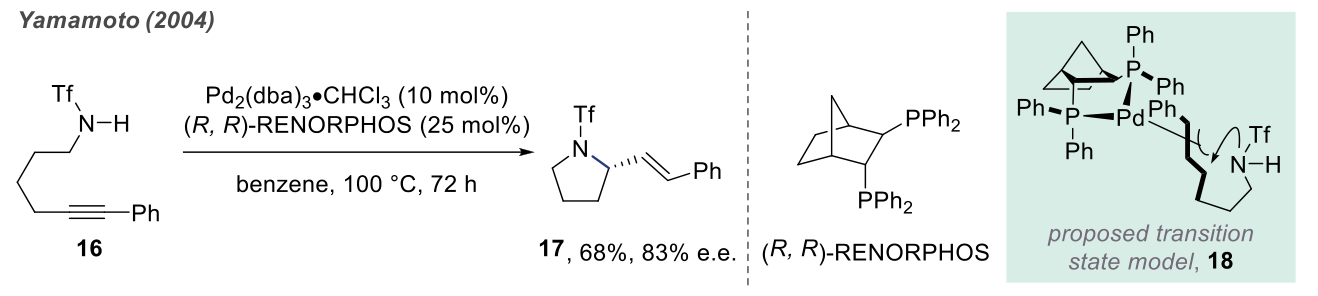
Intramolecular Hydroamination In the presence of transition metal catalysts, the direct introduction of the amine moiety into non-functionalized hydrocarbons is the most ideal method for amine synthesis. Along these lines, hydroamination, the reaction between simple amines and non-activated olefins, represents a particularly attractive and atom-economical pathway for the synthesis of secondary and tertiary amines.
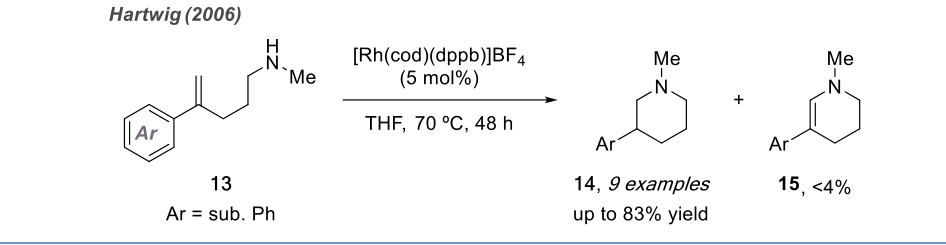
Although the pyridine moiety is highly significant in drugs and natural products, research on the intramolecular anti-Markovnikov hydroamination of unactivated alkenes is still limited due to the competitive oxidative amination reaction that leads to the formation of cyclic imines. In 2003, Hartwig successfully achieved the anti-Markovnikov addition cyclization process through intramolecular hydroamination of unactivated alkenes using rhodium-catalyzed reactions.
Compared to simple olefins, the weaker C=C bond in olefinic compounds (∼10 kcal/mol) provides a potential advantage for asymmetric transition metal catalysis, requiring fewer restrictions. However, due to the linear geometry of auxiliary coordinating ligands, it was previously believed that the use of chiral phosphine ligands was too far from the reaction center to induce chirality.
In 2007, Widenhoefer and Toste independently discovered chiral dinuclear gold complexes that catalyze the asymmetric hydroamination of olefins.
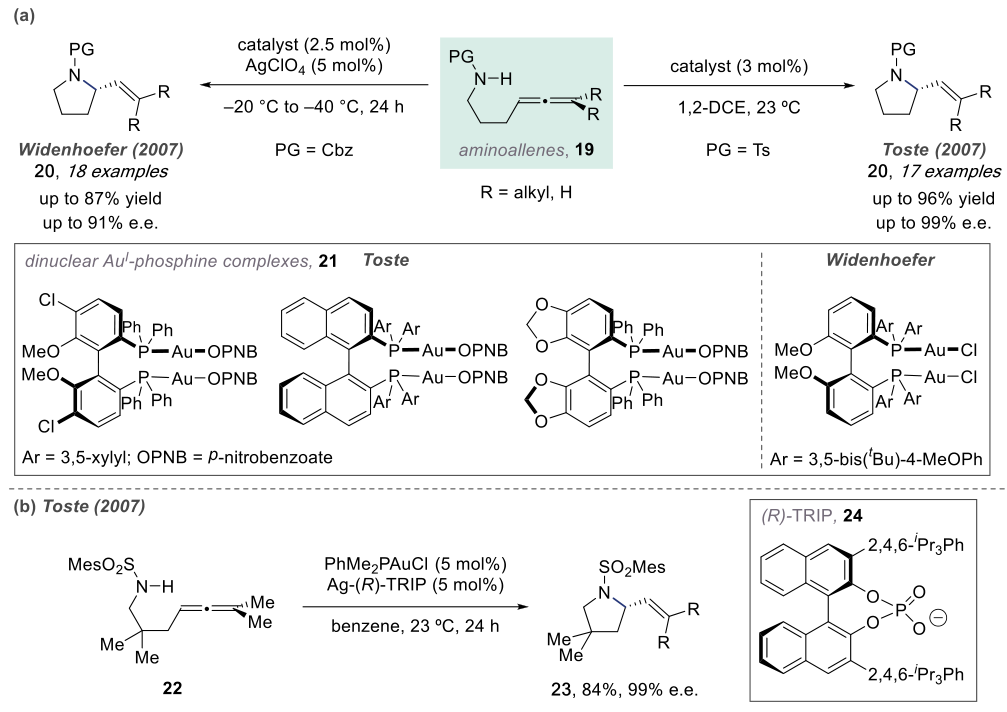
The mechanisms of such reactions are similar. Below, we take the example of a Pt-catalyzed reaction.
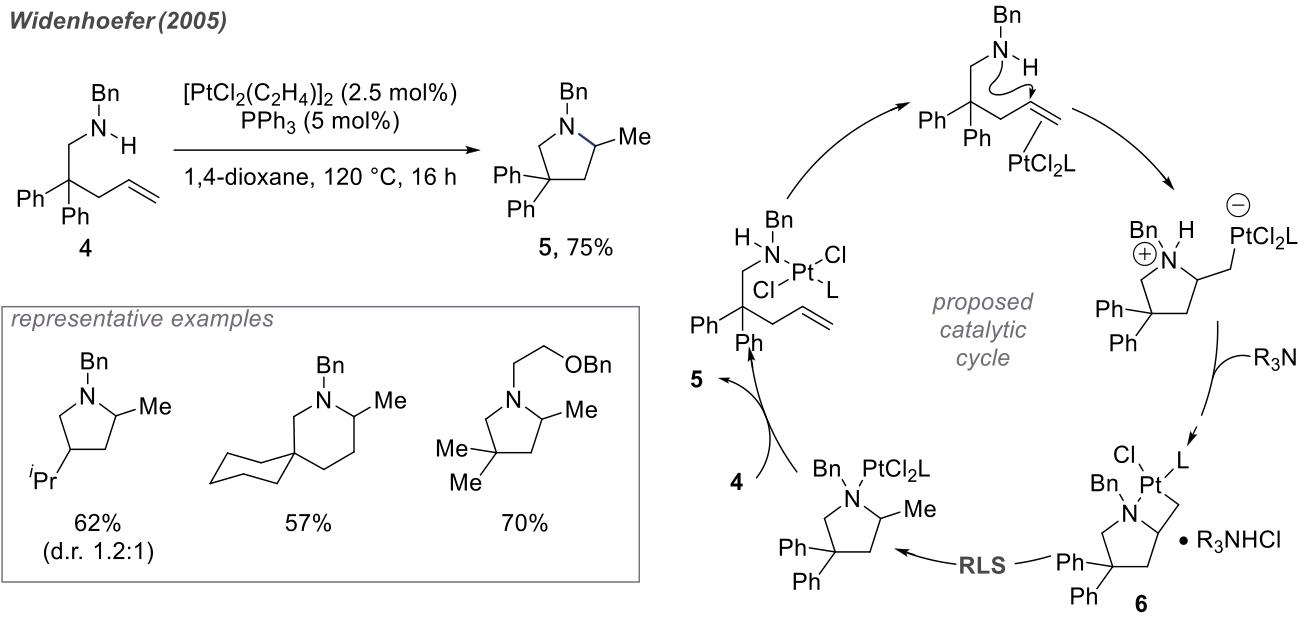
Platinum ions are soft acids, belonging to the same category as the previously mentioned gold ions. In the nucleophilic cyclization of amines, platinum ions act as soft acids. Subsequently, the amine, after losing a proton, undergoes ligand substitution with the platinum atom, forming a four-membered ring with the Pt-C bond as the weakest link. Ultimately, the catalyst re-enters the cycle.
In contrast to typical cyclizations, the advantage of these transition metal-catalyzed reactions lies in the ability to reverse the so-called "polarity" and even alter selectivity by modifying ligands.
Intermolecular Amination Looking solely at the products, intermolecular reactions often result in linear-chain amines. However, the mechanisms vary significantly. Intermolecular reactions require catalysts with high activity when coordinating with olefins. The most commonly used catalysts are not conventional Lewis acids but coupled catalysts such as Pd and Ir. Palladium is introduced into the system as a divalent species and is reduced by a reducing agent to Pd(0), exhibiting enhanced reactivity.
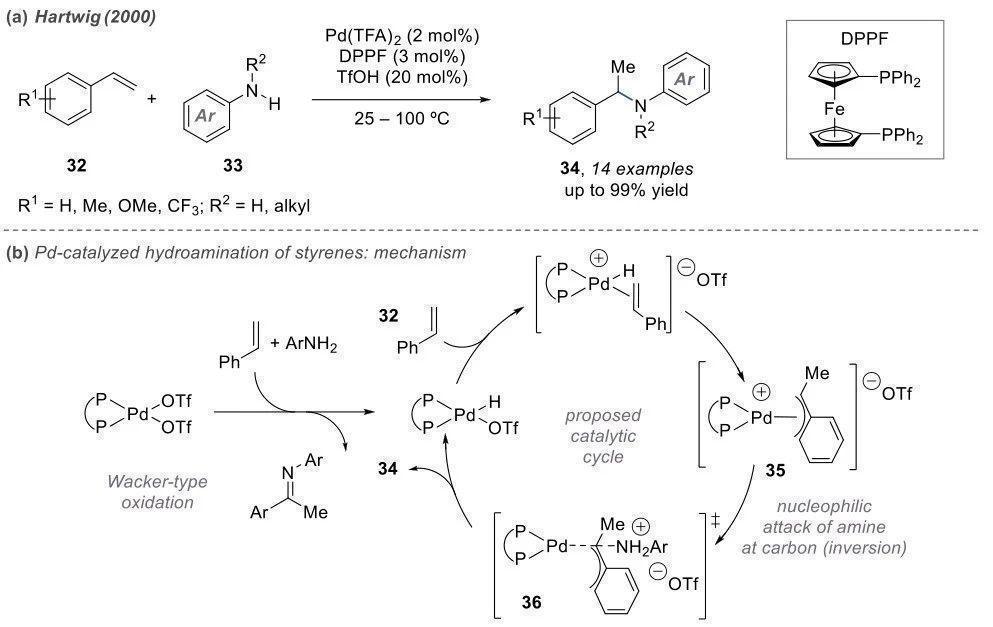
Pd(0) initially coordinates with the double bond, resembling the Heck reaction, leading to insertion. Palladium binds to a portion of the benzene ring, generating a carbon cation equivalent. Subsequently, nucleophilic attack by the amine occurs, and the catalyst re-enters the cycle.
In 2014, Grubbs discovered a two-step, one-pot strategy for the anti-Markovnikov hydroamination of olefins, yielding moderate to good yields. Impressively, this reaction was found to be applicable to the coupling of styrene and some aliphatic olefins with primary and secondary aromatic amines. It is speculated that the reaction proceeds through a Wacker oxidation and hydrogenation amination process catalyzed by iridium.
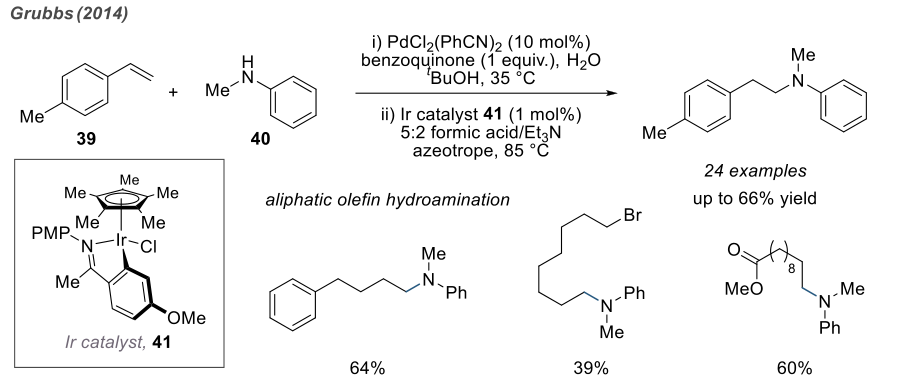
Since there are reactions catalyzed by chiral complexes, there are also reactions induced by chiral ligands. The use of chiral phosphine compounds enables the stereoselective formation of products.
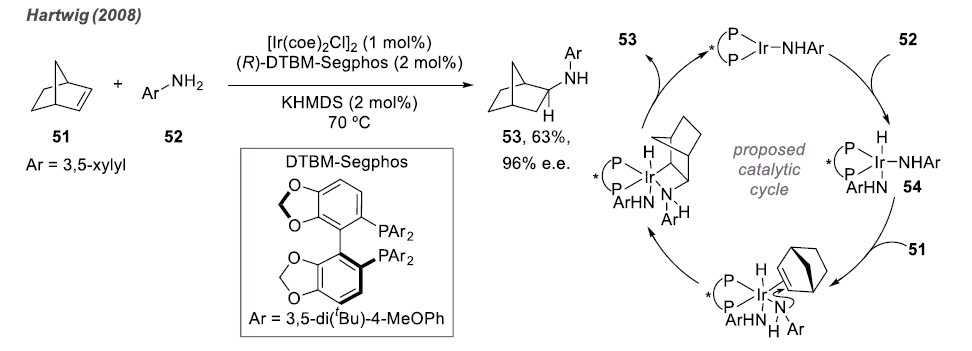
Different from activated olefins, including dienes, pre-coordinated compounds, and aryl olefins, many aliphatic olefins do not exhibit high reactivity. Therefore, intermolecular hydroamination reactions are not as readily achievable as intramolecular reactions.
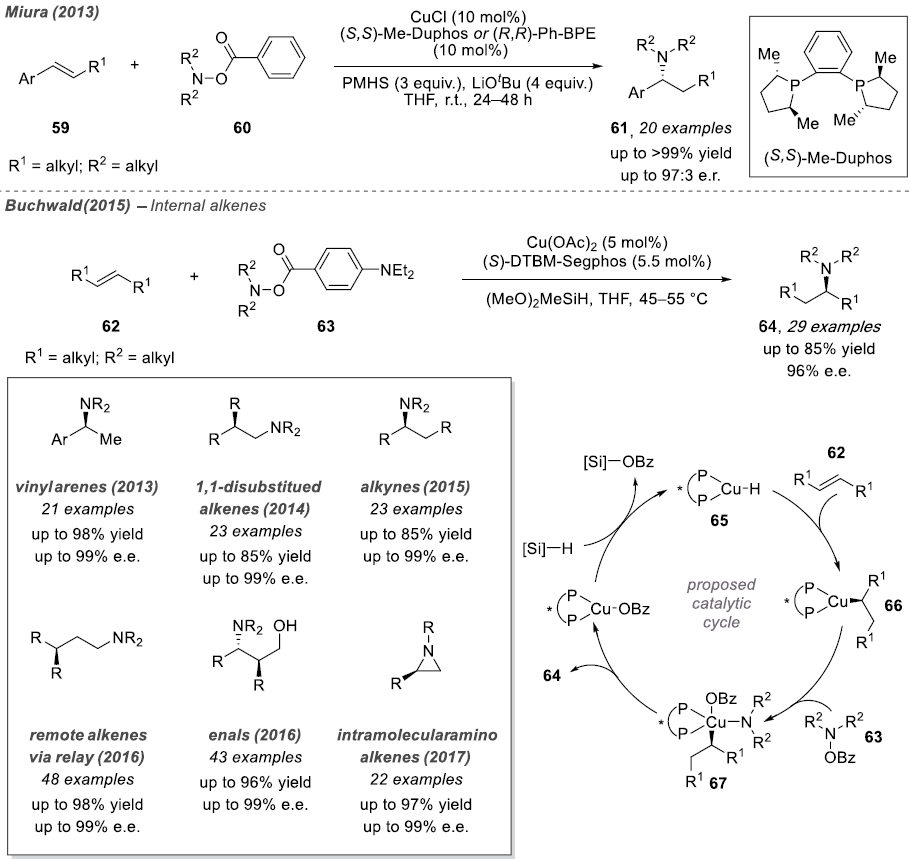
In 2013, both Miura and Buchwald provided detailed descriptions of intermolecular asymmetric hydrogenation reactions, utilizing ortho-benzoic hydrazide and Ph-BPE and DTBM-Segphos ligands as catalysts, respectively. The authors hypothesized that the enantioselective step involves the insertion of the olefin into the Cu-H species. The resulting copper alkyl species undergoes oxidative addition with ortho-benzoic hydrazide to generate a trivalent copper intermediate. Mechanistic studies indicate that the alkyne amination reaction first protonates the vinyl-copper species with an alcohol additive, followed by hydroamination of the generated olefin intermediate.