Despite variations in the structures of surfactants, they share common fundamental properties—adsorption and aggregation. When dissolved in water, surfactants easily adsorb (accumulate) at the air/water interface, forming a neatly arranged monolayer film (Figure 2(a)). In addition to the air/water interface, surfactants can also accumulate at the oil/water interface, reducing interfacial tension and altering the structure and properties of the oil/water interface film. With the assistance of surfactants, oil and water can form emulsions, widely used in daily life and industrial and agricultural production. They can also adsorb onto solid surfaces, enhancing the wetting properties of solid substrates. The reduction of surface/interface tension through adsorption is one of the fundamental properties of surfactants.
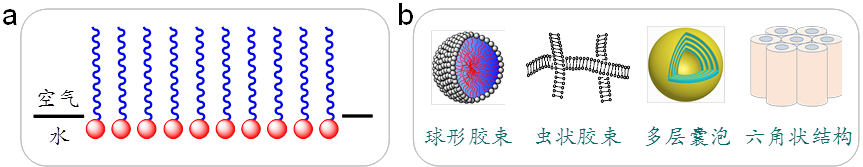
Figure 2 Schematic representation of surfactant adsorption at the air/water interface forming a monolayer film (a) and typical aggregate structures formed in a surfactant aqueous solution (b). In (b), a portion of the spherical micelle and vesicle is removed to reveal the internal structure. For better illustration, the sizes of the monolayer film and various aggregates are not presented to scale according to the actual proportions of the same surfactant.
When there is an excess of surfactants and saturation adsorption is reached at the air/water interface, surfactants tend to aggregate in the bulk phase. Since most surfactants first aggregate into micelles, this concentration is known as the critical micellar concentration (cmc). The types of aggregates formed by surfactants are diverse and closely related to the structure and concentration of the surfactant, as well as external conditions. Typical examples include spherical, rod-like, disc-shaped, and worm-like micelles; single-layer or multi-layer vesicles; lamellar, hexagonal, cubic liquid crystals; and gels containing three-dimensional networks. Some representative types are schematically depicted in Figure 2(b). This aggregation process, being spontaneous, is also referred to as self-assembly. These structures are crucial subjects of study in the fields of soft matter and nanotechnology. Moreover, the spontaneous transition from disorder to order in this self-assembly process, contrary to the law of entropy increase, gives it significant scientific research value.
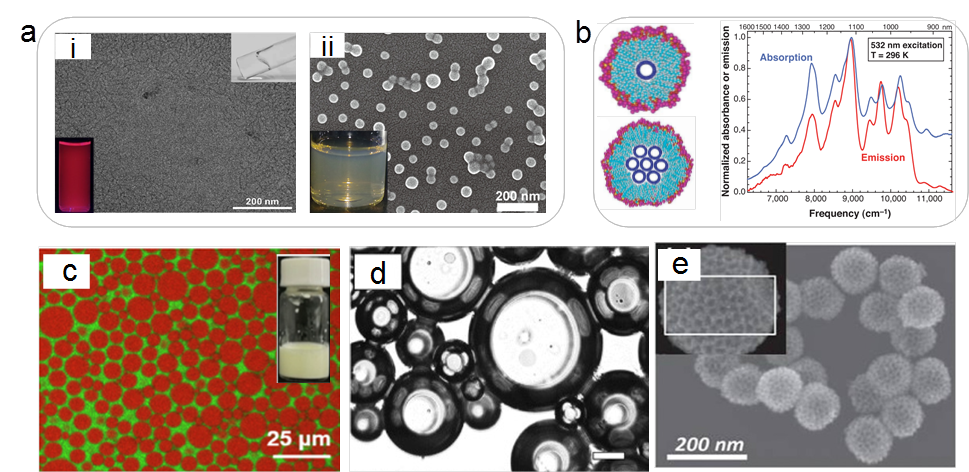
Figure 3 Typical functions of surfactants, using hydrocarbon surfactants as an example. (a) Solubilization. Solubilization of a rare earth complex in a worm-like micelle formed by an amphoteric surfactant (i) and solubilization of fullerene C60 in a block copolymer micelle (ii). (b) Anionic surfactant-assisted dispersion of single-walled carbon nanotubes: model (left) and fluorescence spectra of the dispersion (right). (c) Emulsification of toluene-water system using alkyl glucoside; the water phase is colored green, and toluene is colored red. (d) Super-grade extra virgin olive oil foam stabilized by octadecyl sucrose ester. (e) Pt-Ru nanoparticles formed by the synergistic action of Pluronic F127 as a soft template and silica nanoparticles as hard templates.
The basic properties of surfactants, such as easy adsorption and self-aggregation, give rise to a variety of functions. The hydrophobic tails of surfactants can insert into oil contaminants, reducing the oil-water interface tension. With the assistance of mechanical stirring, they can further solubilize the contaminants, forming swollen micelles or emulsions. This mechanism underlies the process of cleaning, playing a crucial role in various applications of surfactants. In daily life, surfactants are widely present in various cleaning products such as laundry detergents, dish soaps, and shampoos, serving as core ingredients in these products. In industrial and agricultural production, from vehicle cleaning to tertiary oil recovery agents, surfactants are utilized for their cleaning properties.
In cutting-edge research, the same principles are employed to dissolve structurally complex, sparingly soluble substances in water, forming homogeneous and stable solutions. Examples include rare earth complexes and fullerene C60 (Figure 3a). When the size of the insoluble guest is large, surfactants cannot facilitate complete dissolution but can only encapsulate its surface, providing dispersion and stability, as seen in the case of one-dimensional, hydrophobic carbon nanotubes (Figure 3b). Examining these processes from an energetic perspective reveals that surfactants effectively reduce the solid/liquid interface energy. Similarly, the amphiphilic nature of surfactants ensures their adsorption at liquid/liquid interfaces, reducing the interfacial energy, which is commonly exploited in emulsification processes.
Taking the system in Figure 3c as an example, where water (green) and toluene (red) are immiscible, the addition of alkyl glucoside surfactant reduces the interfacial energy between toluene and water. This means that even with an increased area of the liquid/liquid interface, the system can stably exist, allowing toluene to exist in the form of small-sized droplets within the water phase, forming a stable emulsion. Similarly, when insoluble gases are dispersed (encapsulated) in a liquid, a foam system can be formed, and the reduction of interfacial energy (surface tension) by surfactants positively contributes to enhancing foam stability. Thanks to advancements in surfactant science, foam technology has expanded from the aqueous phase to the oil phase, achieving high-performance foamability and foam stability. Recent work (Figure 3d) demonstrates the excellent foaming performance and high-temperature stability of octadecyl sucrose ester in an extra virgin olive oil foam system, with potential applications in food science.
Within surfactant solutions, after surfactants form micelles, they can serve as excellent soft templates. These structures are not only uniform and stable but also easy to remove. They play a crucial role in the synthesis of inorganic semiconductor quantum dots, silica nanoparticles, and molecular sieves. Interestingly, surfactant soft templates can synergistically work with hard templates such as silica nanoparticles. For instance, in the preparation of hollow, mesoporous noble metal materials, surfactants can be co-loaded with noble metal salts on the surface of hard templates, providing mesoporous templates for the subsequent formation of noble metal particles. Such mesoporous metal nanoparticles, formed using this method, exhibit high specific surface area and are excellent electrocatalysts (Figure 3e). The interiors of micelles formed by surfactants often exhibit a non-(micro)polar state. When a small amount of nonpolar component is introduced into a surfactant solution, it can be enveloped by the micelles, forming thermodynamically stable microemulsion systems. Besides their high stability, microemulsions also possess optically transparent characteristics. Therefore, microemulsions play an irreplaceable role in drug loading, colloidal optics, and other fields.
At higher surfactant concentrations, different types of liquid crystals can be formed, known as lyotropic liquid crystals. As soft materials with long-range ordered structures, liquid crystals combine the fluidity of liquids with the orderliness of crystals. Materials synthesized using liquid crystals as templates often exhibit good controllability and plasticity in terms of structure, attracting extensive attention in the fields of optics, life sciences, materials science, and cosmetic science.
The application of surfactants in biological systems is also widely explored. Sodium dodecyl sulfate (SDS), anionic surfactant, serves as an auxiliary component in protein separation gel formulations. Research on the use of surfactants as drug carriers, high-molecular-weight phenolic surfactants as bioadhesives, environmentally responsive surfactants for preparing smart soft materials, and surfactant-assisted genetic engineering is seamlessly integrating ancient surfactants with the "century of biology." In recent years, surfactants have excelled in fields such as flexible electronic devices, fuel cells, efficient proton exchange membranes, and energy-saving pollution reduction. Additionally, some surfactants also possess multiple functions such as sterilization and antistatic properties. The diverse performance of surfactants makes their applications extensive. Apart from daily life, cleaning,
Despite the significant achievements in surfactant science, there are still some shortcomings. The discovery of novel surfactants has expanded their types, but there is still a long way to go in correctly selecting and applying them in specific scenarios. Many structurally novel surfactants have a limited variety of hydrophilic and hydrophobic groups, and further exploration is needed. The preparation routes for these surfactants also need optimization to reduce costs. A comprehensive understanding of the performance of many new surfactants will also take time.
The use of surfactants faces challenges. With the increasing awareness of environmental protection, the environmental and biological compatibility of surfactants has become a topic of concern. While fluorinated surfactants possess unique properties, their retention in the human body limits their applications. The degradation products of alkylphenol polyethylene glycol ethers have been found to pose potential harm to humans. The excessive release of phosphorus-containing surfactants can lead to the overgrowth of aquatic plants, disrupting the normal life of aquatic animals. When cationic surfactants enter the human body, they can easily bind with phosphate groups in cell membranes, thereby interfering with normal cell functions. Therefore, the development of environmentally and biologically friendly surfactants is an important research direction in the field. Natural (biomass) surfactants are highly anticipated in this regard. Surfactants originating from nature are expected to return to nature.
In this era, surfactant science is experiencing unprecedented development opportunities. How to leverage strengths, avoid weaknesses, reconcile differences, and promote the ancient yet youthful discipline is a challenge for scientists, especially colloid and interface chemists. With the continuous efforts of scientists in various fields and the ongoing development of contemporary technology, it is believed that surfactant science will soon overcome challenges, achieve new brilliance, and continue to play a crucial role in human production and life.